Laboratory of Immunology
Home | Research Focus | Research Activities | Staff | Contact | Publications and Patents
- At the Laboratory of Immunology, we are involved in two major research activities: diagnostic technology and snake venom research.
Diagnostic technology
- We focus on developing simple diagnostic tests for the detection of infectious agents and/or their corresponding specific antibodies. Examples of research activities include:
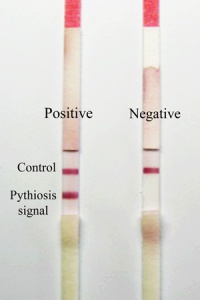
Immunochromatographic test for rapid diagnosis of human pythiosis
Human pythiosis is an emerging and life-threatening infectious disease caused by the fungus-like organism Pythium insidiosum. It is endemic in Thailand where about 80% of the cases are reported. The disease has high rate of morbidity and mortality, due to lack of early diagnosis and effective treatment. We have developed a convenient and reliable immunochromatographic test (ICT) for rapid detection of human anti-P.insidiosum IgG, for use in early diagnosis and monitoring of pythiosis patients, resulting in saving of limbs and lives. The test kit has been mass produced for free distribution to 6 hospitals with medical schools throughout the country. This research has been published and a patent application has been submitted. In collaboration with researchers from the Department of Internal Medicine, Siriraj Hospital and the Department of Pathology, Ramathibodi Hospital, the ICT has been used to study the seroprevalence of P.insidiosum infection in high-risk hematological patients, and a multi-host ICT for the detection of P.insidiosum infection in human, horse and dog has been developed.
Improvement in sensitivity of tests for detection of Vibrio cholerae O1 and O139
Cholera, a major public health burden in developing countries, is caused by two epidemic strains of Vibrio cholera (VC) O1 and O139. Immunochromatographic tests (ICTs) have been developed for both strains for rapid detection in stools to replace the conventional time-consuming culture method. However, the ICTs are so far not sensitive enough to detect the low numbers of bacteria found in carriers, so we have modified the structure of the specific lipopolysaccharides (LPS) to allow for more specific interaction with antibody and thereby increase the sensitivity.
Rapid diagnostic test to detect specific microbial nucleotide sequences
Recently, there is an urgent need for rapid diagnostic tests for new infectious agents in man and animals. Conventionally, these infectious agents are identified either by culture or by using molecular biology techniques, e.g. PCR. These processes are costly and usually take several hours or even days. We have produced luminol encapsulated biotinylated liposome and used it as a generic signal generator, capable of conjugation to biotinylated biomolecules, such as specific microbial oligonucleotides, antibody and antigen. The system has been used to detect specific oligonucleotide hybridization and specific antigen-antibody interaction, and the results have been published.
Snake venom research
- We have been involved in biochemical, pharmacological and immunochemical studies on snake venoms, and examples of current research activities are shown below.
Production of a pan-specific antivenom against elapid snakes
Snake venoms are produced by snakes for digestion and to rapidly immobilize their prey. Venoms contain various hydrolytic enzymes and toxins e.g., neurotoxins and cytotoxins. Neurotoxic venoms are mainly produced by elapid snakes (cobra, krait, mamba) and sea snakes. These toxins cause neuromuscular blockage, leading to respiratory failure and death. Postsynaptic neurotoxins (PSNTs) from different elapids, although chemically and pharmacologically similar, are immunochemically distinct, so antivenom (AV) against venom from one elapid usually fails to neutralize venom of another elapid, even from the same genus. Thus, each country or region needs to produce its own AV, which is not economically or technically always possible. The lack of effective AVs has resulted in numerous deaths of snakebite victims. Our aim is to produce a pan–specific antivenom against venoms of Southeast Asian elapid snakes.
Previously we have established an immunization protocol (WHO Guidelines, 2010), which is highly effective in producing high titer and high affinity antibody against PSNTs of various snakes. Potent polyspecific antivenom has been produced against 3 elapids from Thailand with ED50s comparable to the corresponding monospecific antivenoms. Using this immunization protocol with selected neurotoxins from various elapids, we are studying the preparation of a pan-specific antivenom against elapids of SE Asian countries. If successful, this antivenom could be produced for wide distribution and, with economy of scale, should be quite cheap, widely available and save the lives of snakebite victims in many countries.
Local injection of enzyme inhibitors/antibody for prevention/reduction of tissue necrosis
The venoms of the Malayan pit viper (Calloselasma. rhodostoma, CR) and the Thai cobra (Naja kaouthia, NK) can cause systemic as well as local poisoning. While specific antivenoms can reverse systemic toxicity, they do not reduce local effects, which can lead to prolonged hospitalization and sometimes amputation. This important problem has been studied in rodents, whose skin structures are very different from man. So we are studying local tissue necrosis (LTN) in the pig, searching for a possible treatment modality.
We have shown that intradermal, subcutaneous and intramuscular injections of CR venom into a pig (20 kg) could be used for quantitation of intradermal induration, subcutaneous hemorrhage and myonecrosis. Moreover, it was possible to study the histopathology of LTN by taking biopsies at various times after venom injection, and various distances from the injection site. This provided useful clues to the possible causative agents (enzymes, toxins), which lead to LTN, indicating that the pig can serve as a good experimental model for LTN. In addition, various enzyme inhibitors (N-phenylglycine for metalloprotease and phospholipase A2 and sodium aurothiomalate for hyaluronidase) and equine F(ab΄)2 antibody against CR, when injected 2 minutes after and into the venom injection site, could reduce induration, subcutaneous hemorrhage and plasma creatinine kinase. Histopathology studies indicate that injection sites also showed reduction of LTN.