Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 2411
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 1496
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 1496
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 999
Strict Standards: Only variables should be assigned by reference in /opt/lampp/htdocs/dokuwiki/inc/JpegMeta.php on line 1496
Laboratory of Medicinal Chemistry
Home | Research | Academic | Publications | Staffs | Contact
Research Activities
Development of Methodology-Driven Cutting-Edge Synthetic Technologies
 The current paradigm shift in organic chemistry has prompted us to devise and develop novel, efficient, practical, and economical synthetic routes with low environmental impact. The use of catalysts, microwave energy, and subcritical water in the reactions conform well to some of the important “green chemistry” principles which emphasize on high efficiency of the reactions, utilization of an alternative source of cleaner energy, and the use of water as solvent. These important considerations have played a crucial role for the successful development of C-H activation chemistry as well as cyclization reactions to form some pivotal scaffolds of natural products via reactive intermediates such as quinone methides. In addition, various solid-supported reagents have been utilized in organic reactions to improve chemical yields and selectivities. These “reagents on the beads” offer some significant advantages over the conventional reagents as they exhibit greater compatibility with and tolerability over a range of functional groups and substrates, as well as their recyclability.
The current paradigm shift in organic chemistry has prompted us to devise and develop novel, efficient, practical, and economical synthetic routes with low environmental impact. The use of catalysts, microwave energy, and subcritical water in the reactions conform well to some of the important “green chemistry” principles which emphasize on high efficiency of the reactions, utilization of an alternative source of cleaner energy, and the use of water as solvent. These important considerations have played a crucial role for the successful development of C-H activation chemistry as well as cyclization reactions to form some pivotal scaffolds of natural products via reactive intermediates such as quinone methides. In addition, various solid-supported reagents have been utilized in organic reactions to improve chemical yields and selectivities. These “reagents on the beads” offer some significant advantages over the conventional reagents as they exhibit greater compatibility with and tolerability over a range of functional groups and substrates, as well as their recyclability.
Target-based Synthesis of Bioactive Compounds for Future Drug Development
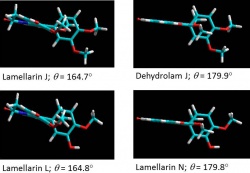 Due to the rare natural abundance of most biologically active compounds, total synthesis is a vital alternative to provide sufficient quantities of compound as well as allow structural modifications for establishing their pharmacological profiles while minimizing toxicity. Our interest in the lamellarins, a group of marine natural products exhibiting anticancer, anti-HIV-1 integrase, and multidrug-resistance reversing activities, has led us to design and synthesize over 100 structurally related derivatives of lamellarins. In addition, preliminary structure-activity relationships have been established. Currently, our effort has been focused on streamlining the structural requirements of lamellarins while incorporating functional groups to enhance aqueous solubility and assessing the drug-likeness.
Due to the rare natural abundance of most biologically active compounds, total synthesis is a vital alternative to provide sufficient quantities of compound as well as allow structural modifications for establishing their pharmacological profiles while minimizing toxicity. Our interest in the lamellarins, a group of marine natural products exhibiting anticancer, anti-HIV-1 integrase, and multidrug-resistance reversing activities, has led us to design and synthesize over 100 structurally related derivatives of lamellarins. In addition, preliminary structure-activity relationships have been established. Currently, our effort has been focused on streamlining the structural requirements of lamellarins while incorporating functional groups to enhance aqueous solubility and assessing the drug-likeness.
Complementary to the total synthesis approach for bioactive compounds, we have also contemplated semi-synthesis approach to prepare analogs of some valuable medicinally useful natural products such as the huperzines, a group of naturally occurring alkaloids from firmoss Huperzia serrata.